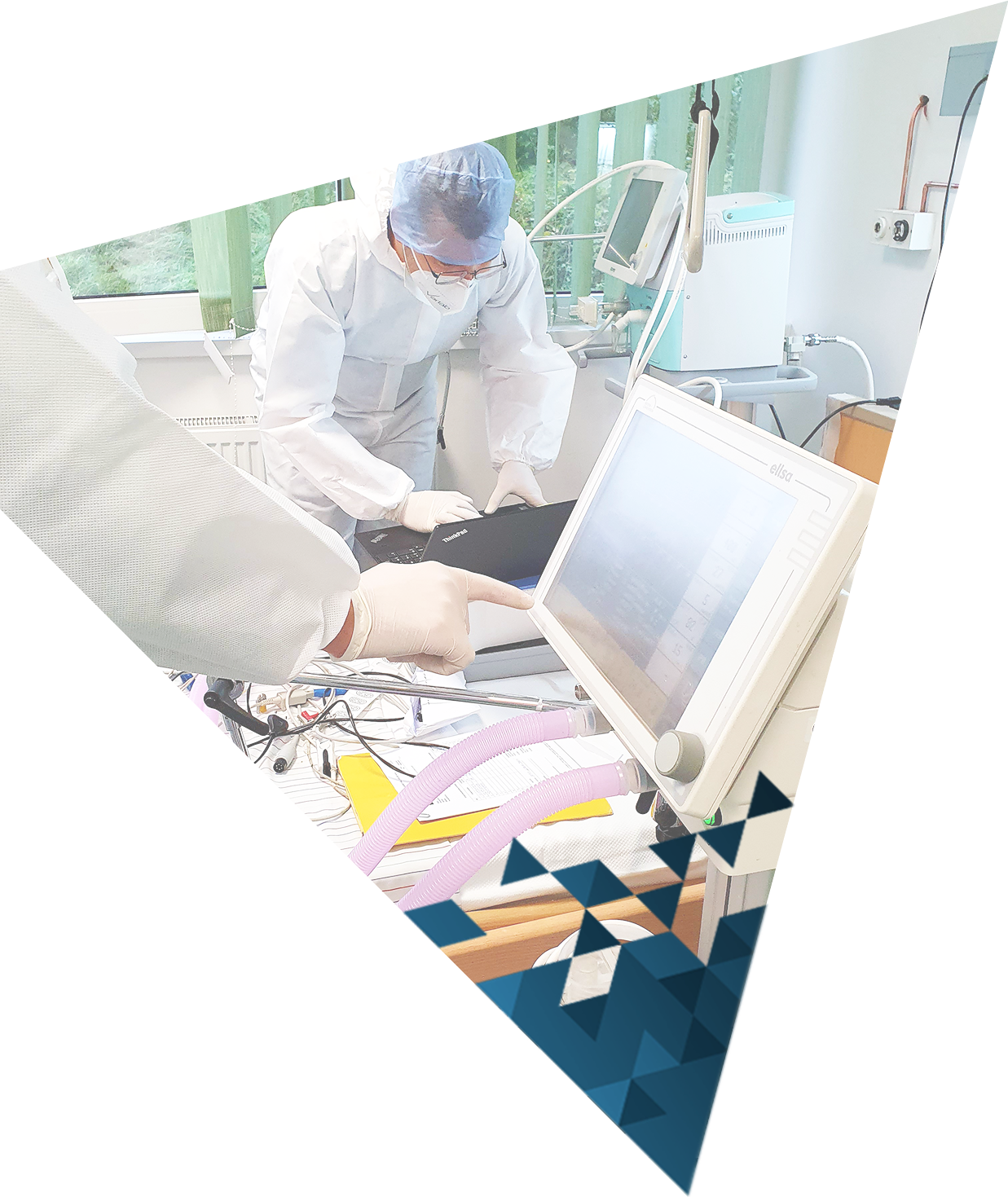
Verlab is a company based in Southeast Europe that provides services within biomedical and clinical engineering. We cooperate with international organizations and national regulatory bodies.
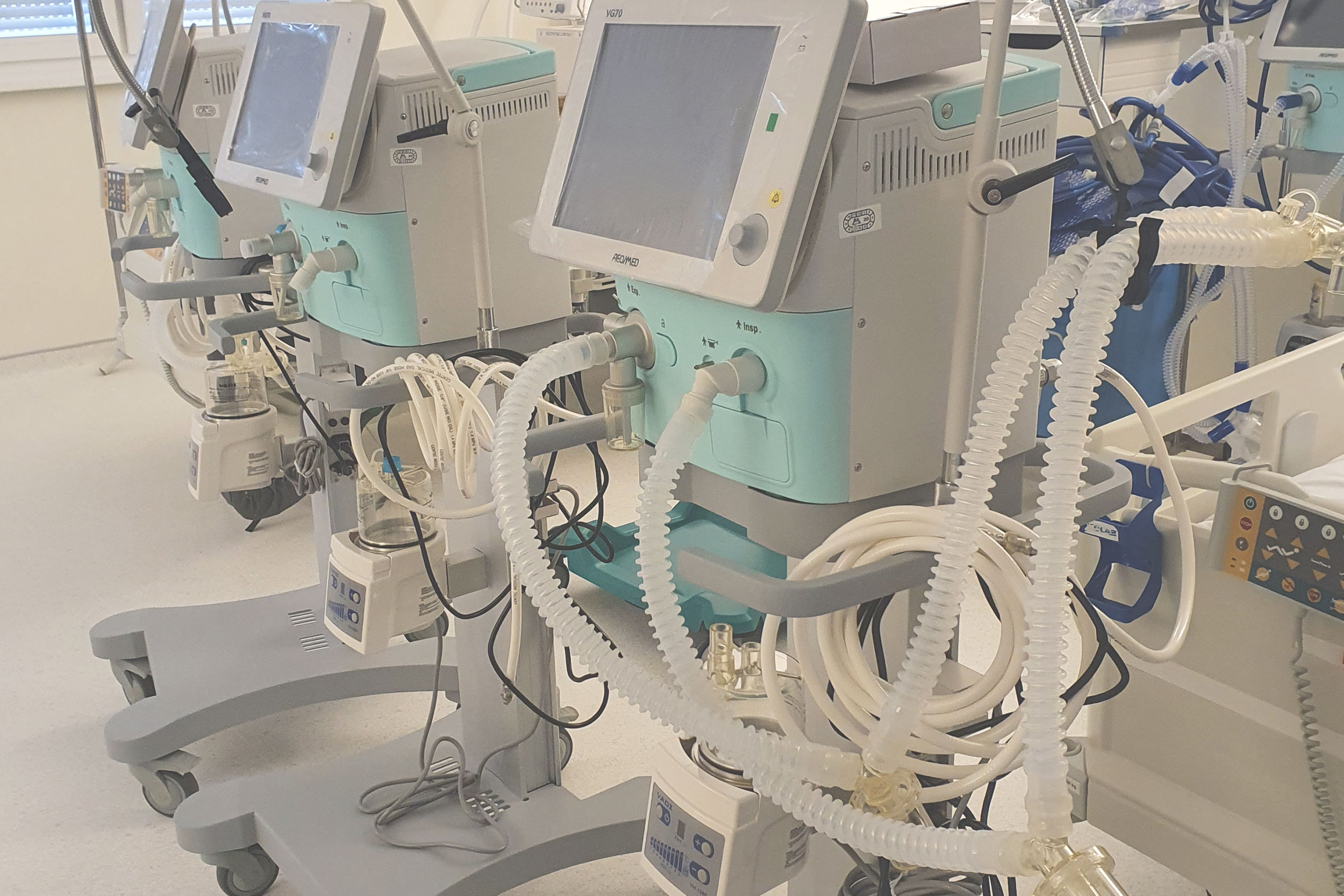
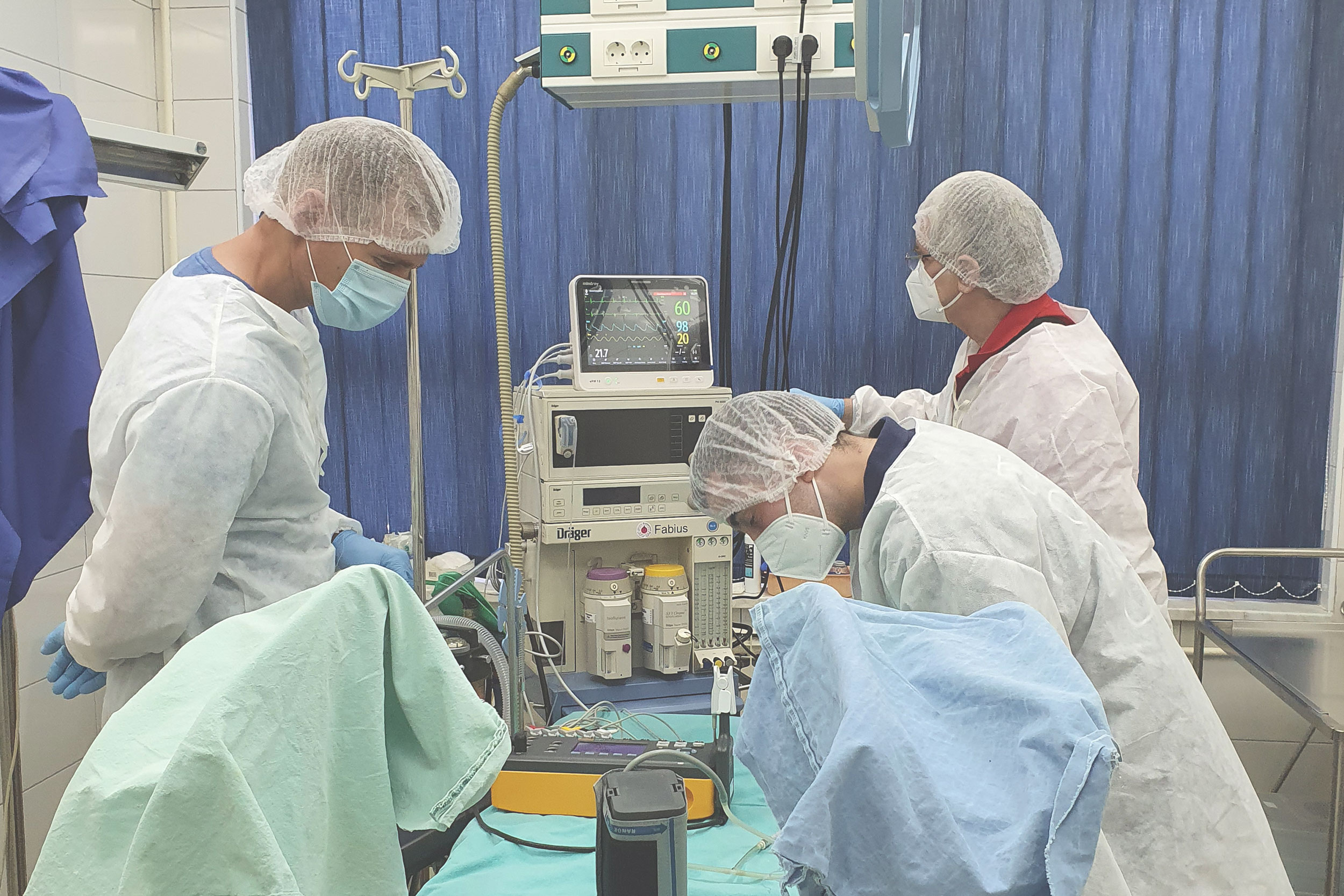
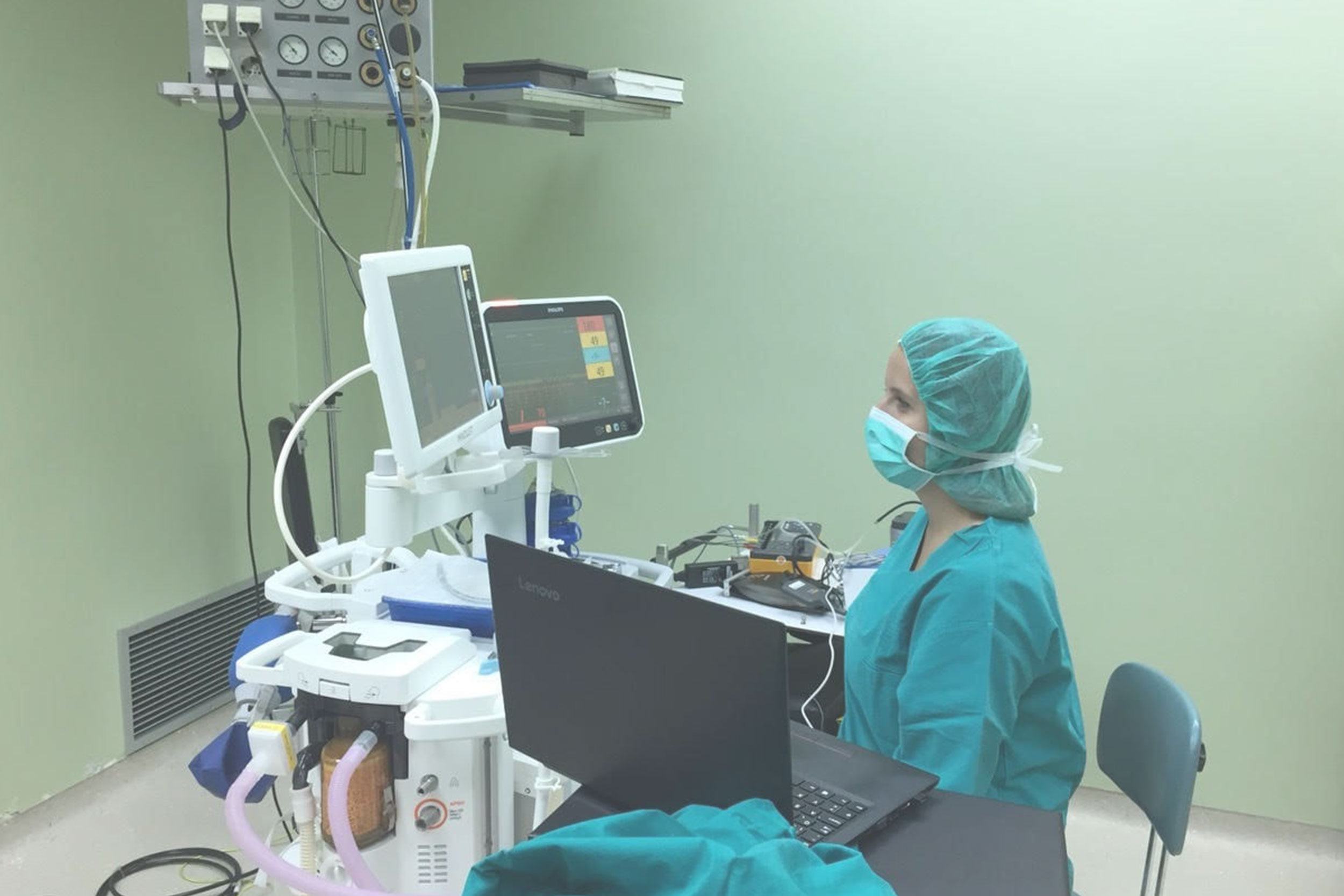
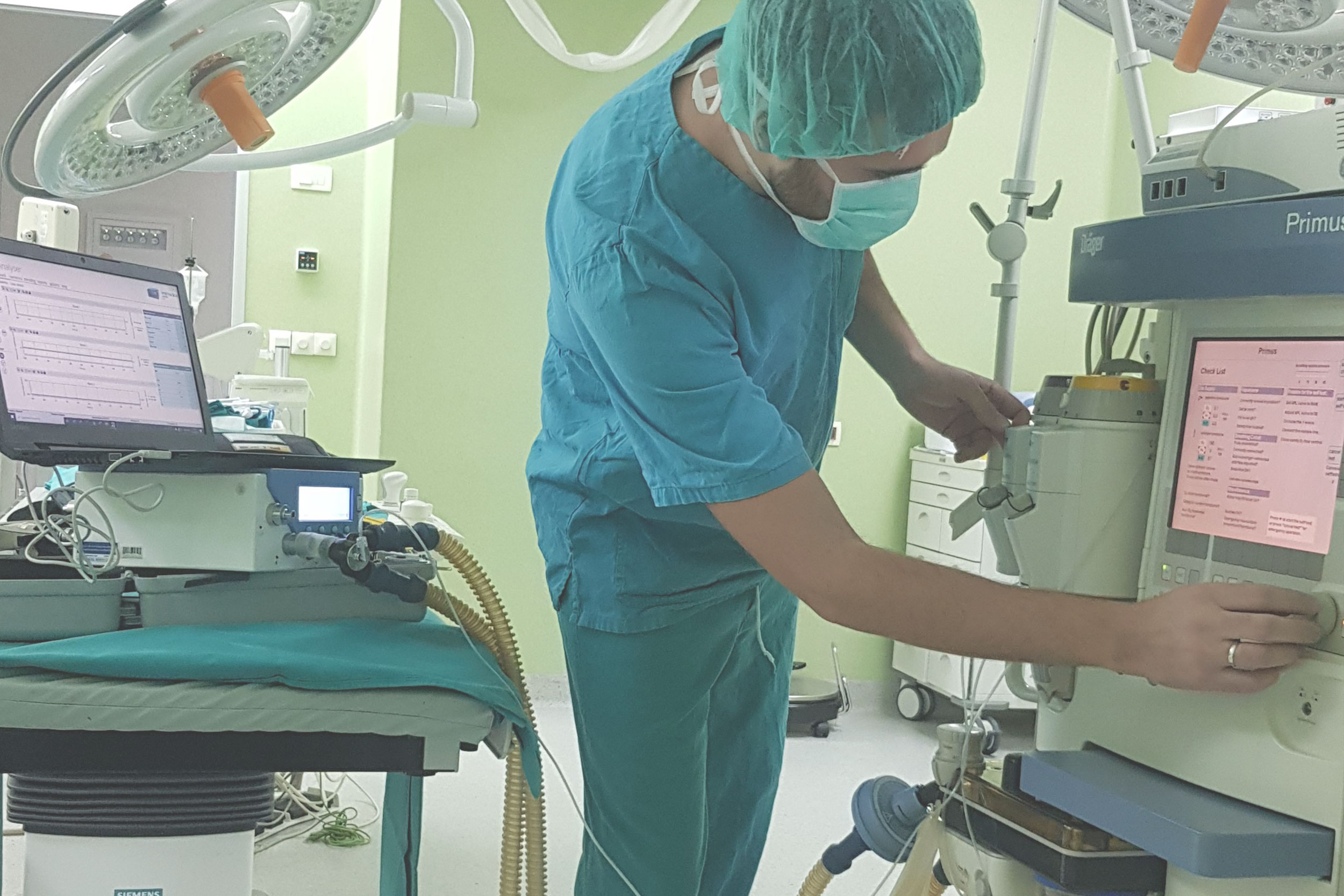
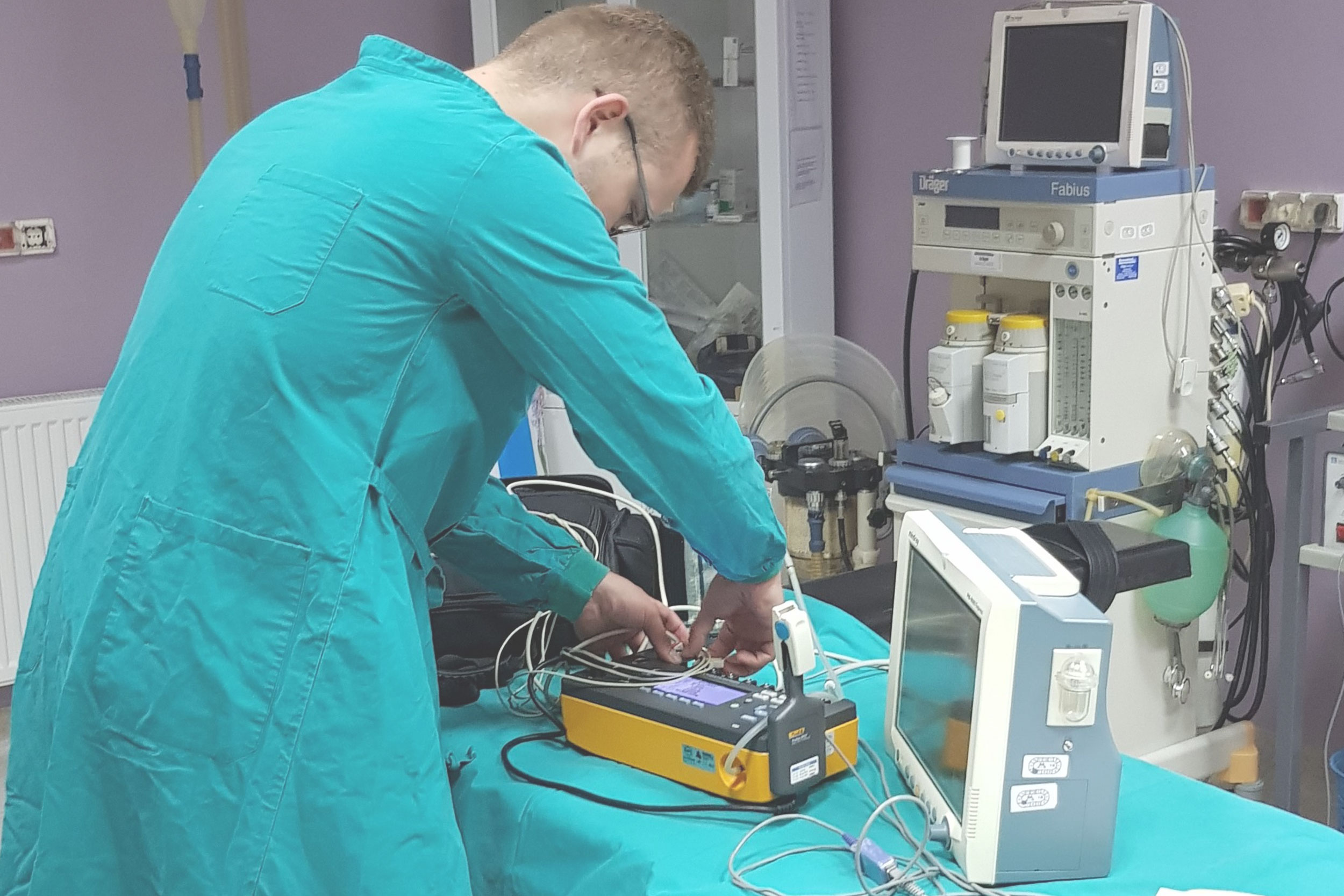
Medical Device Inspection Laboratory within Verlab provides services of verification of medical devices with measuring function that are part of the legal metrology in accordance with the Law on Metrology in Bosnia and Herzegovina. Medical devices with measuring function for which verification/inspection activities are performed are: ECG devices, defibrillators, patient monitors, incubators for pediatric and neonatal patients, anesthesia machines, mechanical ventilators, infusomats and perfusors, therapeutic ultrasound devices, dialysis machines and blood pressure monitors.
Medical Device Inspection Laboratory is appointed by the Institute of Metrology of Bosnia and Herzegovina in accordance with the Law on Metrology in Bosnia and Herzegovina and accredited inspection body inspection / control body according to the requirements of the standard BAS EN ISO 17020. Accreditation was performed by the Institute for Accreditation of Bosnia and Herzegovina.
Verlab also provides services of development of digital platforms in healthcare and consultations in the field of biomedical and clinical engineering.
Verlab’s five core values – impartiality, objectivity, integrity, excellence and innovation – are more than just words. They are the foundation of our culture. These principles guide us in our work together to advance the quality of care in the health sector.
Our dedication to research & innovation resulted in establishment of the first private research institute in Bosnia and Herzegovina. Through our Verlab Research Institute we work on generating, applying, and disseminatin research findings through a global ecosystem of forward-looking stakeholders to build capacity by empowering the next generation of technology-enabled professionals.
MEET
OUR TEAM
Our employees are awarded by IFMBE Clinical Engineering Division Award for distinguished contribution of an article advancing the global Clinical Engineering field with impact on mankind’s healthcare services.